PPT The Arrhenius Equation PowerPoint Presentation, free download
This calculator calculates the effect of temperature on reaction rates using the Arrhenius equation. k=A*exp (-Ea/R*T) where k is the rate coefficient, A is a constant, E is the activation energy, R is the universal gas constant, and T is the temperature (in kelvin). R has the value of 8.314 x 10 -3 kJ mol -1 K -1.
Apply the Arrhenius equation to calculate how many ti… SolvedLib
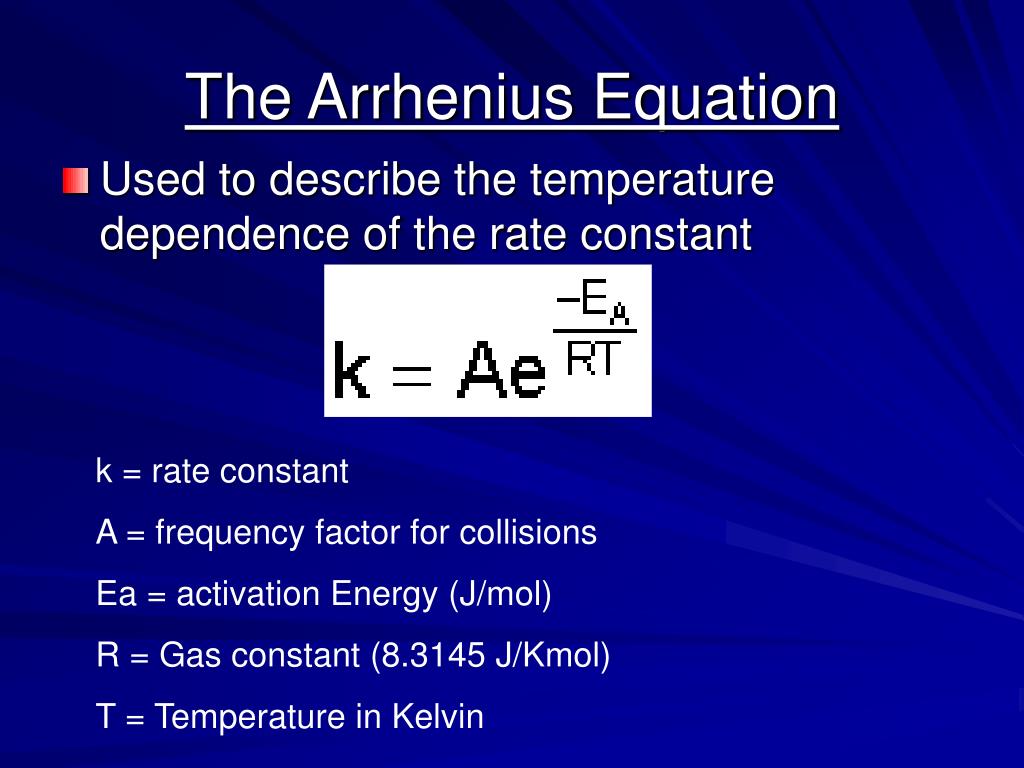
The Arrhenius equation is a simple and accurate formula for the temperature dependence of the chemical reaction rate constant. The Arrhenius equation can be used to show the effect of a change of temperature on the rate constant and on the rate of the chemical reaction. k = A*e (-Ea/RT) A = pre-exponential or frequency factor.

16.2 The Arrhenius equation (HL) YouTube
Arrhenius Equation Calculator - Free Online Calculator Learn how to use the Arrhenius equation calculator with a step-by-step procedure. Get the Arrhenius equation calculator available online for free only at BYJU'S. Login Study Materials NCERT Solutions NCERT Solutions For Class 12 NCERT Solutions For Class 12 Physics

Arrhenius Equation YouTube
The exponential term in the Arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. Because the rate of a reaction is directly proportional to the rate constant of a reaction, the rate increases exponentially as well.. Calculate the activation energy if the pre-exponential.
[Solved] Using Arrhenius equation to calculate the ratio 9to5Science
The Arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the A factor (also known as the pre-exponential factor; can be visualized as the frequency of correctly oriented collisions between reactant particles).
Worked Example with the Arrhenius Equation YouTube
The Arrhenius equation, from a chemical point of view, is: k = A\cdot e^ {\frac {-E_ {\text {a}}} {R \cdot T}} k = A ⋅ e R⋅T −Ea We can identify many elements in the Arrhenius equation: k k is the rate constant, usually expressed in the units \text {M}^ {1-n}\text {s} M1−ns, where n n is the reaction order;

Arrhenius Equation
The Arrhenius equation is used to calculate the rate constant (k) of a chemical reaction as a function of temperature (T), activation energy (Ea), and the pre-exponential factor (A). The equation is expressed as: k = A * exp (-Ea / (R * T)) Where: k is the rate constant A is the pre-exponential factor or frequency factor Ea is the activation energy

Arrhenius Equation Calculator Formula Example Calculator Academy
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the rate constant, and therefore rate, of a chemical reaction. At higher temperatures, the probability that two molecules will collide is higher.
PPT The Arrhenius Equation PowerPoint Presentation, free download
The Arrhenius equation Forms of the Arrhenius equation Using the Arrhenius equation Collision theory and the Maxwell-Boltzmann distribution Elementary reactions Reaction mechanism and rate law Reaction mechanism and rate law Multistep reaction energy profiles Catalysts Types of catalysts Types of catalysts Science > Chemistry library > Kinetics >

The Arrhenius Equation (A Level Chemistry) Teaching Resources
The Arrhenius equation gives the dependence of the rate constant of a chemical reaction on the absolute temperature as where k is the rate constant (frequency of collisions resulting in a reaction), T is the absolute temperature (in Kelvin or degree Rankine ), A is the pre-exponential factor or Arrhenius factor or frequency factor.
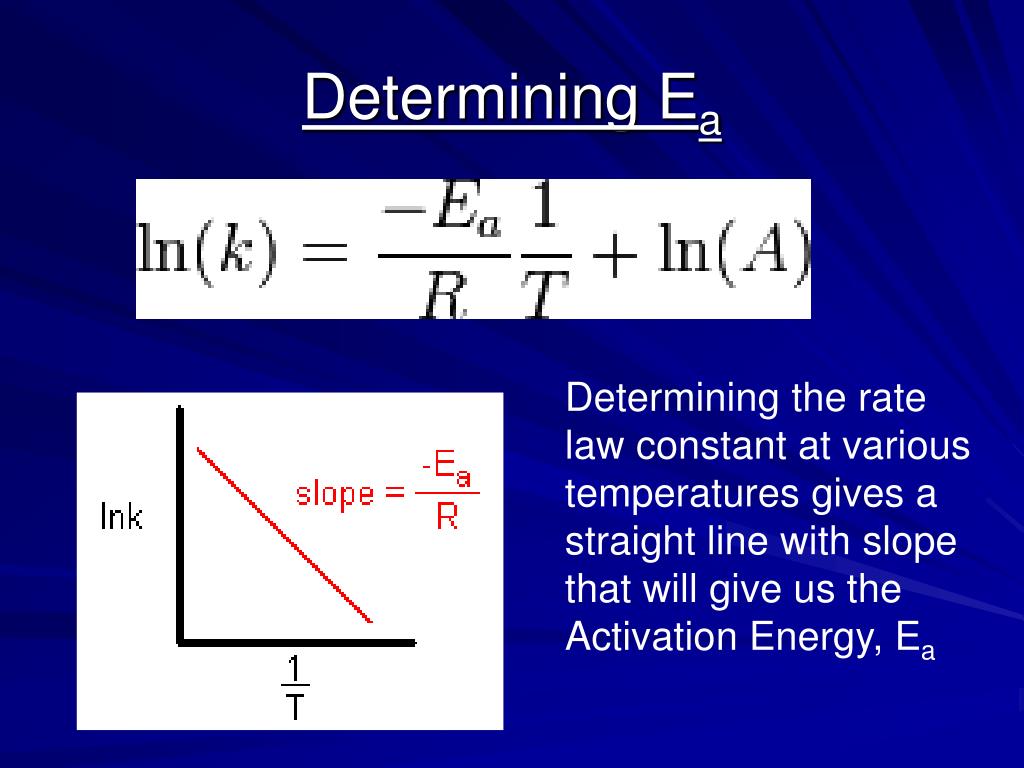
PPT The Arrhenius Equation PowerPoint Presentation, free download
The Arrhenius Equation Calculator is used to compute the frequency factor of a chemical reaction. The user must know the rate constant, activation energy, and the temperature at which the reaction is taking place. The Arrhenius Equation comes from the collision theory of molecules.

Arrhenius Equation
The Arrhenius equation is used to determine the activation energy or rate constant of a chemical reaction. as the temperature changes. If you want to see how the rate constant changes when the temperature changes, the Arrhenius equation is your friend.. Calculate the energy of activation for this chemical reaction. Solution: Since we are.
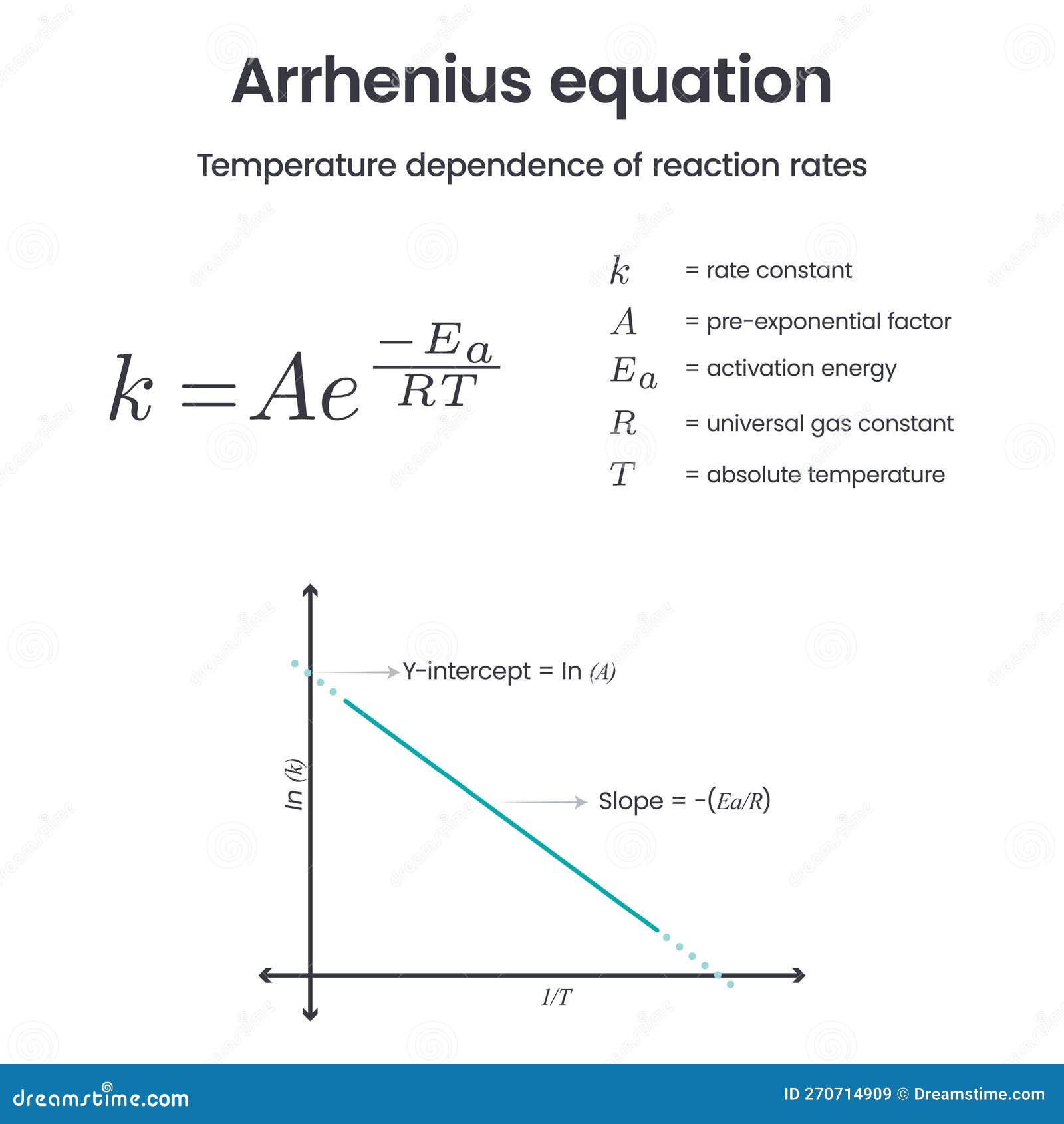
Arrhenius Equation Physical Chemistry Science Vector Infographic Stock
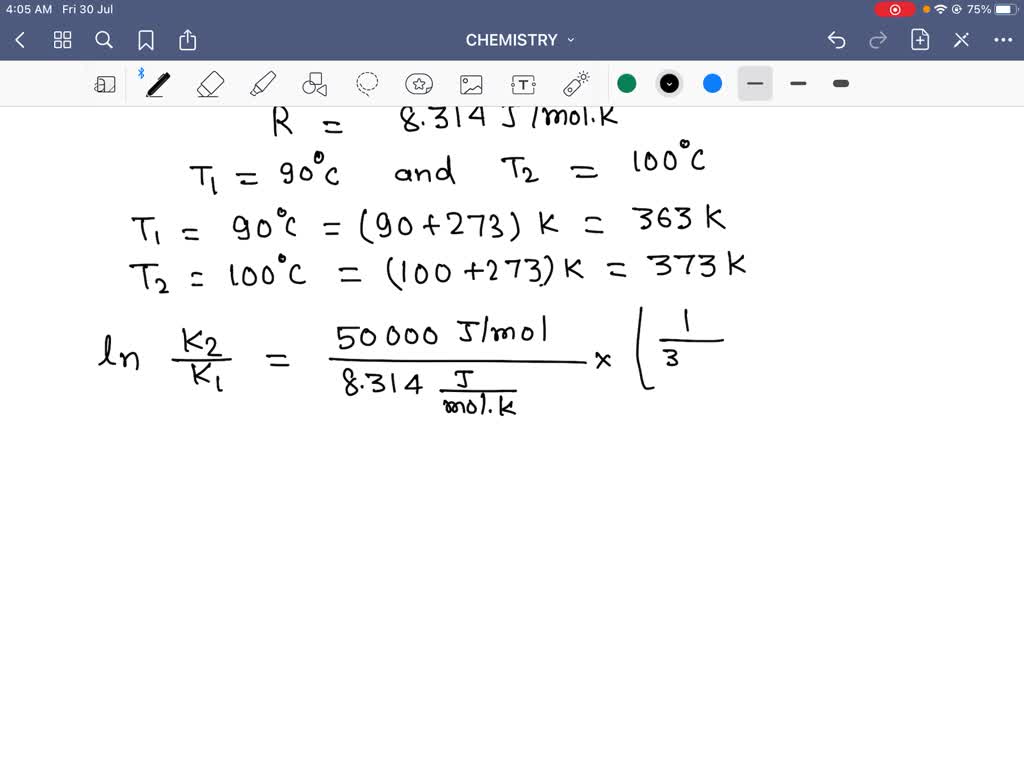
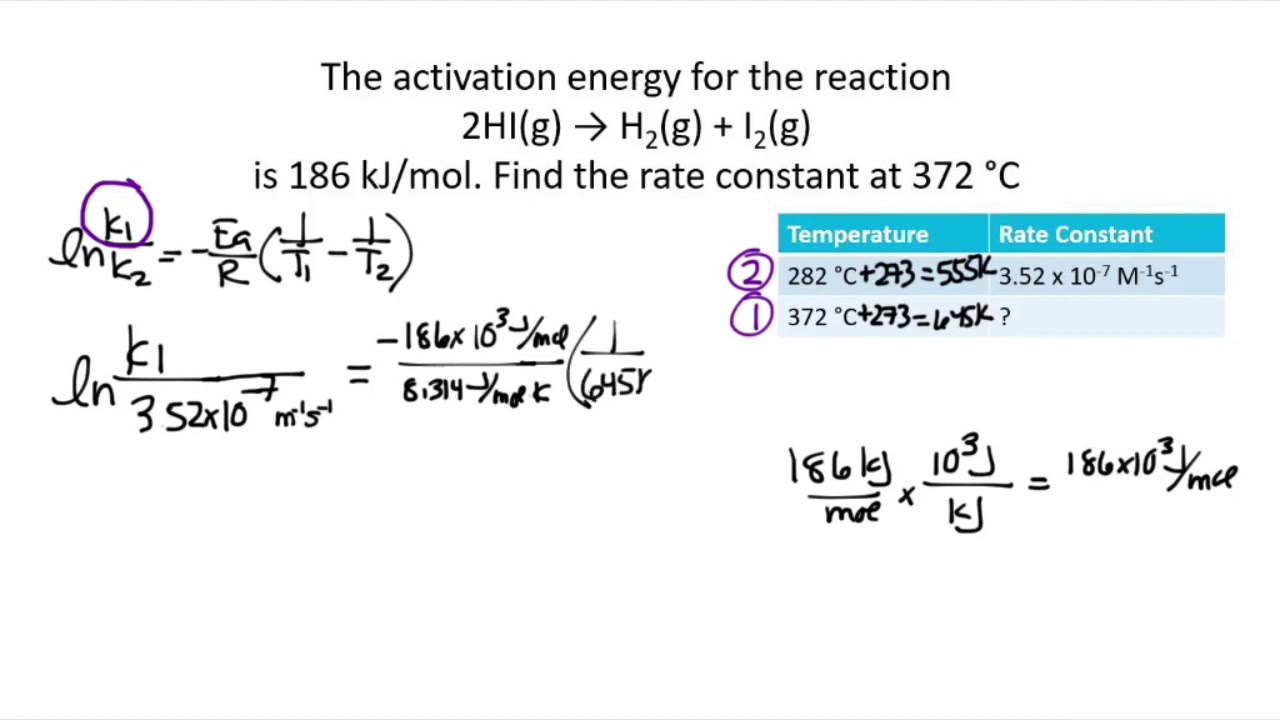
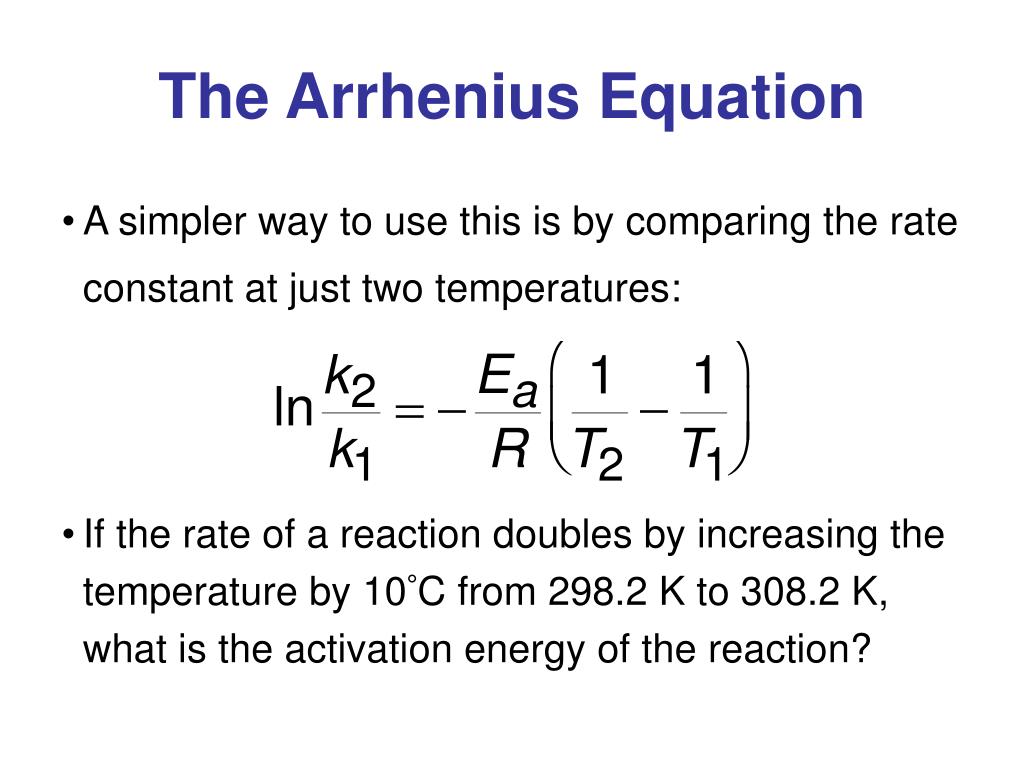
The Math / Science The Arrhenius Equation, k = A⋅ e− Ea RT k = A ⋅ e - E a RT, can be rewritten (as shown below) to show the change from k 1 to k 2 when a temperature change from T 1 to T 2 takes place. The Activation Energy equation using the Arrhenius formula is: Ea = R⋅ ln(k2 k1) 1 T 1 − 1 T 2 E a = R ⋅ ln ( k 2 k 1) 1 T 1 - 1 T 2 where:

Arrhenius Calculations YouTube
The Arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. If we look at the equation that this Arrhenius equation calculator uses, we can try to understand how it works: k = A\cdot \text {e}^ {-\frac {E_ {\text {a}}} {R\cdot T}}, k = A ⋅ e−R⋅T Ea, where:
30+ arrhenius equation calculator MujahidTaylore
You can find the activation energy for any reactant using the Arrhenius equation: E_\mathrm {a} = -R × T × \ln\biggl (\frac {k} {A}\biggr) E a = −R × T × ln(Ak) where: R R — Gas constant. It is equal to \mathrm {8.314\ J/ (K\!\cdot\!mol)} 8.314 J/(K⋅mol); T T — Temperature of the surroundings, expressed in Kelvins; k k — Reaction rate coefficient.
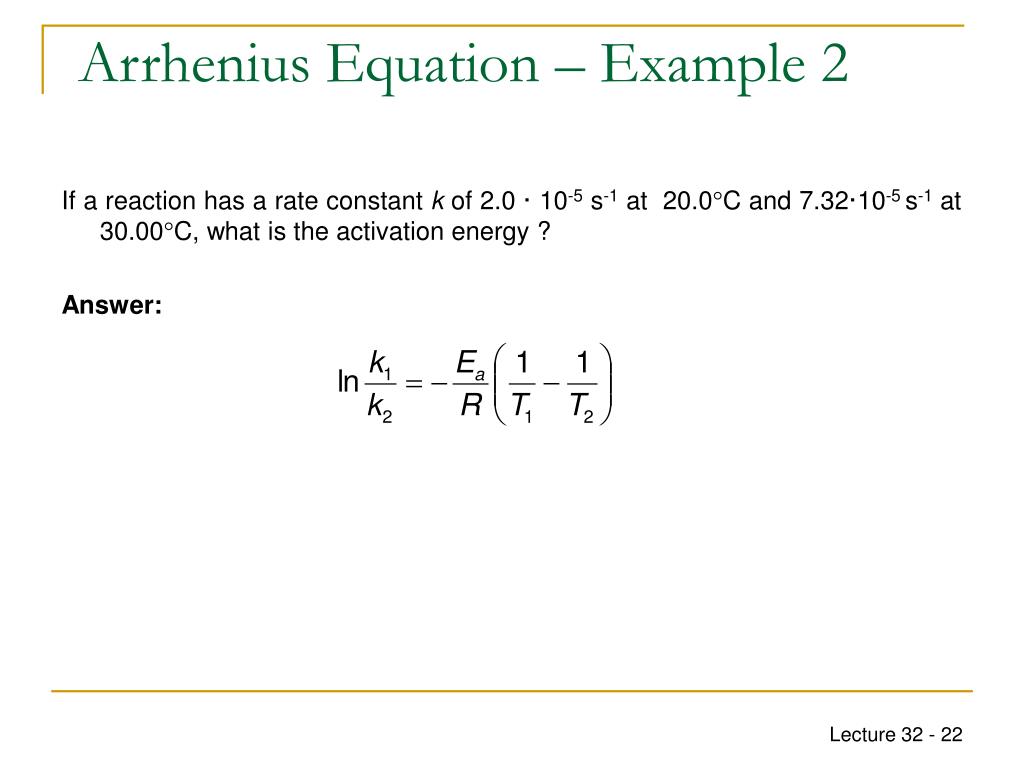
PPT CHEM1612 Pharmacy Week 11 Arrhenius Equation
The 'Arrhenius Equation Calculator' is a free online tool that calculates the rate constant in the Arrhenius equation for a reaction. In this calculator, you can enter the Activation Energy (Ea), Temperatur, Frequency factor and the rate constant will be calculated within a few seconds. How to Use Arrhenius Equation Calculator?